[01/09/2019] Effectiveness and safety of intravitreal aflibercept in patients with wet age- related macular degeneration treated in routine clinical practices across France: 12-month outcomes of the RAINBOW study
All the publications
BMJ Open Ophthalmology 2019;4:e000109.
background/aims To monitor treatment-naïve patients with wet age-related macular degeneration (wet AMD) receiving intravitreal aflibercept (IVT-AFL) in France. Methods RAINBOW (Real life use of intravitreal Aflibercept In FraNce - oBservatiOnal study in Wet age- related macular degeneration) is an ongoing, observational, retrospective and prospective 4-year study to assess visual (primary), anatomical and safety outcomes following IVT- AFL treatment in wet AMD patients. We report the interim 12-month outcomes in patients who have already been enrolled.
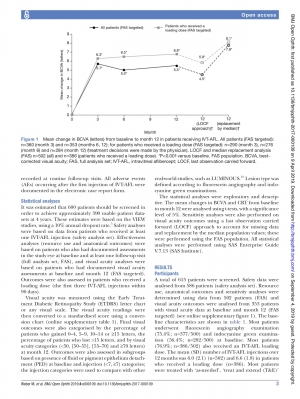
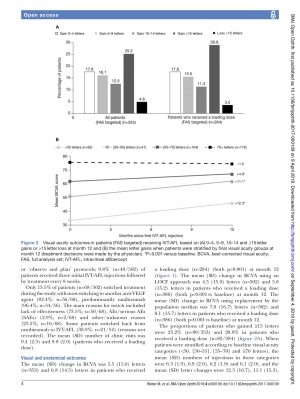
results Safety data were analysed from 586 patients (safety analysis set); and effectiveness data were analysed from 502 patients with at least one follow-up (full-analysis set) and from 353 patients with visual acuity data at baseline and month 12. The mean (SD) best-corrected visual acuity (BCVA) was 56.7 (18.2) letters and the mean (SD) central retinal thickness (CRT) was 395.6 (140.5)
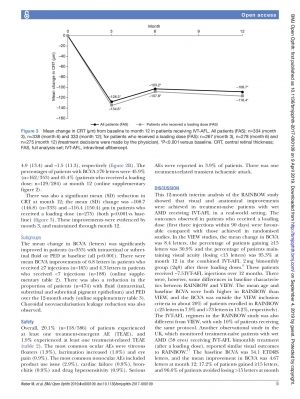
μm at baseline. Most patients (76.9%) received a loading dose (first three injections within 90 days). The mean (SD) number of IVT-AFL injections over 12 months was 6.0 (2.1) and 6.6 (1.8) (patients who received a loading dose). The mean (SD) change in BCVA was 5.5 (15.0) letters and 6.8 (14.5) letters (patients who received a loading dose) at month 12 (p<0.001 vs baseline). The mean (SD) CRT reduction was –108.7 (146.8)μm and –116.4 (150.4)μm (loading dose) at month 12 (p<0.001 vs baseline). Overall, 118 (20.1%) patients experienced at least one treatment- emergent adverse event (TEAE), 1.2% experienced ocular TEAEs and 3.9% experienced serious AEs.
Conclusion This 12-month interim analysis showed that IVT-AFL was associated with sustained improvements in
a real-world setting. The RAINBOW results are consistent with the VIEW clinical studies.
Trial registration number NCT02279537 Pre-results.

 Book an appointment
Book an appointment